Info
ISO/IEC 17025 certified inhalation testing laboratory.
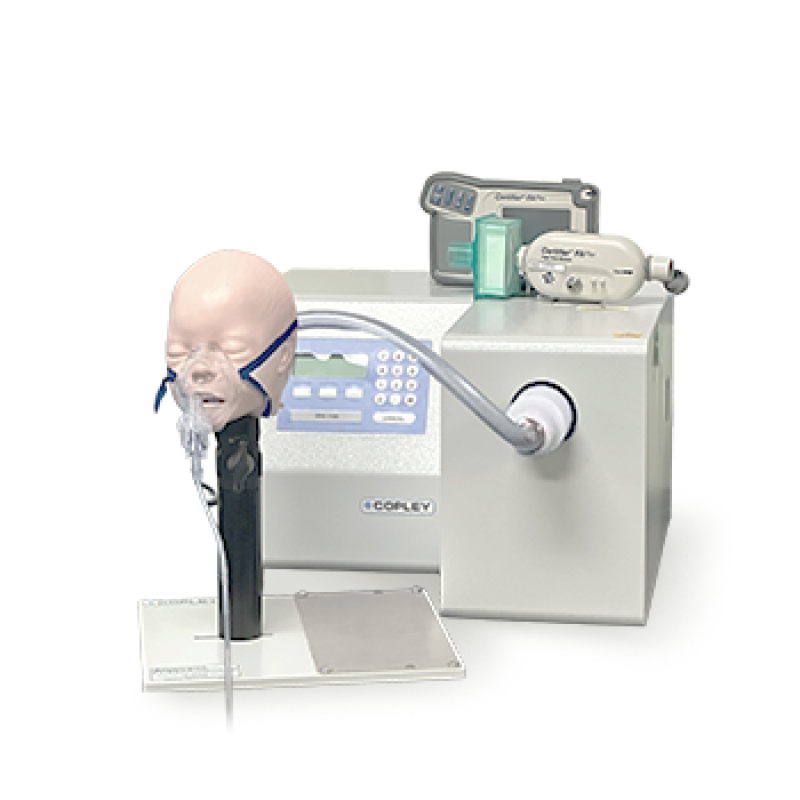
ISO/IEC 17025 certified inhalation testing laboratory.

We provide customized analytical solutions based on industry guidelines, international standards, pharmacopeias (e.g. Ph. Eur., USP, ChP), and customer requirement. Our team of experts built validated methodologies to accurately acquire data and insight into your device performance.
Experienced in regulatory compliance testing and submission in international markets. We work closely with our clients to ensure both efficiency and the smoothest path to regulatory approval.
We provide integrated solution services to support the commercialization of your inhaled device, ranging from aerosol characterization to drug delivery quantification and submission for regulatory approval.
Accreditation
MicroBase Analytic Lab is a fully ISO/IEC 17025 laboratory for performance testing of inhaled product.
USP <1601>
EP <2.9.44>
ISO 27427
EN ISO 27427
EN 13544-1